SYNSTAT®Series Specialised Mattresses

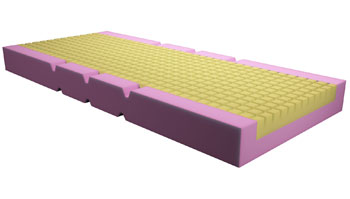
High resilience and medium, medium-high density, static and reactive mattresses manufactured in polyurethane expanded foam, for ordinary, specialised and at risk of decubitus lesions hospitalization. The padding material is alveolar structured in order to ensure high breathability. Our Synstat® series mattresses come as one-block bi-modular structure with incisions on flexion points or as three or more sections.
The base module is manufactured with a ‘cradle’ pattern and with containment edges in expanded polyurethane, polyether type 40 S, high density (40 kg/m3) and high bearing capacity, in order to allow adequate support and safety to the patient.The truncated-pyramid shaped leaning module is manufactured with cellular polyurethane (Synstat® Comfort) or with our special thermo-press-formable technopolymer Synergel® (Synstat® Antidecubito).
Available with Flame Proof Trevira® CS cover (breathable and self-extinguishing) or with Sycura® cover (water-proof, breathable, bielastic, antistatic, self-extinguishing and self-decontaminating).
- Silent, hypo-allergenic, non-toxic, odourless. Do not generate or contain dust or powder particles
- Allow CPR function
- Permanent sanitation treatment to avoid the proliferation of bacteria, fungi and mould
- Can be sanitized, disinfected, sterilized through adequate procedures (padding and covers)
- Latex-Free and radiotranslucent
Available in four versions: Synstat® Comfort, Synstat® Antidecubito,Synstat® 3D,Synstat® Touch Antidecubito.
Certifications
- The performance requirements referred to non-deformability and durability of the Synstat® series mattresses are certified in compliance with UNI 10707 rev.2003 regulations, by LAPI, an authorized laboratory (accordance during technical tests has been achieved overcoming the highest severity level – 45000 cycles).
- The comfort index (Synstat® Comfort) and the antidecubitus index (Synstat® Antidecubito) are certified in compliance with the Ergocert D28 technical regulations.
- The Synstat® series mattresses are CE marked, as provided by Medical Device class 1 MDR UE 2017/745 and type-approved by the Ministry of Interior in class 1IM for fire reaction, in compliance with CSE RF 4/83 DM 26/06/84 (UNI 9175 and UNI 9175/FA1 DM 03/09/2001).
- Ceritfied as Latex-Free products.